
Laboratory work № 1. Definition OF A BODY DENSITY
|
|
AIM: To calculate the density of a body and errors of direct and indirect measurements.
INSTRUMENTS: Vernier callipers, parallelepiped.
System International units
A system of units, known as the System International (SI) units, is now used for all branches of physics. It is based on the meter as the unit of length, the kilogram as the unit of mass, the second as the unit of time, the ampere as the unit of electric current and the Kelvin as the unit of temperature. The unit of force in this system is the Newton and the unit of energy is the Joule.
For many years the meter (m) was taken as the distance between two lines on a particular platinum-iridium rod at 00 C kept near Paris. It is now defined as the length of a certain number of wavelengths in a vacuum of a particular orange radiation of the krypton-86 atom. Unlike the distance between the marks on the rod, the wavelength of the radiation from an atom is a constant. By definition,
1 m = 1650763.73 wavelengths of the above radiation.
The kilogram (kg) is the mass of a particular solid cylinder made of platinum-iridium alloy kept in Paris, known as the International Prototype Kilogram.
In practice, the following smaller units may also be used. The millimeter (mm), which is 1/1000 m. The centimeter (cm), which is 1/100 m The gram (g), which is 1/1000 kg. The second (s) was formerly 1/86400 th part of a mean solar day. This unit, used by astronomers, has now been replaced by an atomic unit. Atomic clocks are now used as standard clocks. Clock which measured the period of rotation of the earth to an exceptionally high order of accuracy and this showed clearly the irregularity in the rate of rotation of the earth. Cesium or atomic clocks are now used as standard clocks. The second is defined as the time for 9192631770 cycles of vibration of a particular radiation from the caesium-133 atom.
The mass per unit of volume of a substance is called its density:
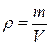 , (4.1)
, (4.1)
where m is the mass of the body and V is its volume. The volume of a parallelepiped equals to:
 , (4.2)
, (4.2)
where a, b, c are the width, the length and the height of the body. The volume of cylinder equals to:
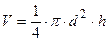 , (4.3)
, (4.3)
where d is the diameter of base of a cylinder; h is the altitude of a cylinder. Thus we can calculate the density of a parallelepiped with the formula:
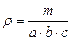 (4.4)
(4.4)
Or the density of a cylinder with the formula:
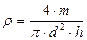 (4.5)
(4.5)
where m is the given mass of the body.
4.2 Volume
Measurements of volume were originally based on the litter; this is defined as the volume occupied by a mass of 1 kg of pure water at its temperature of maximum density and at standard atmospheric pressure. The milliliter (ml) is 1/1000 of a liter. In the SI system, however, volumes are based on the cube whose side is 1 m; that is the cubic meter. This volume is written m3. Volumes are also measured in cubic centimeters (cm3), although this is not an SI unit. 1 cm3 = 1 ml.
Water has a density (mass/volume) of 1000 kg/m3 in SI units (equivalent to l g/cm3); mercury has a density of 13 600 kg/m3 in SI units (equivalent to 13.6 g/cm3).
4.3 Vernier scale
P. Vernier showed how lengths can be measured accurately. He used a subsidiary scale, now called a vernier scale. Fig. 4.1a shows the vernier scale Y below the main scale X, which is graduated in centimeters and millimeters. 10 divisions on Y make 9 mm. Thus the length between each division on Y is 0 ÷ 9 mm, which is 0.1 mm or 0.01 cm less than the length of the divisions on X.
Fig. 4.1b shows the vernier scale Y moved to measure the length of a rod R. X
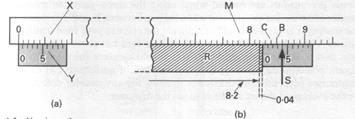
Figure 4.1 – Vernier scale
The length of R is greater than 8.2 but less than 8.3 cm. We now note the division S on Y which coincides with a division on M. This is the fourth division on Y. The third division on Y is thus 0.01 cm in front of the graduation B, the 2nd division on Y is 0.02 cm in front of the graduation C, and so on. Hence the zero of Y is MM cm in front of the 8.2 cm reading on M. Hence the length of R is 8.24 cm.
Fig. 4.2 shows a form of vernier calipers. The object measured is placed between the jaws X and Y, and its length are read from the main and vernier scales as explained above.

Figure 4.2 –Vernier calipers
Micrometer screw gauge
The micrometer screw gauge is an instrument for measuring accurately the diameters of wires or thin rods. It uses an accurate screw of known 'pitch' such as 0÷5 mm, shown inset in Fig. 4.3. This means that for one revolution of the screw the spindle X moves forward or back 0÷5 mm.
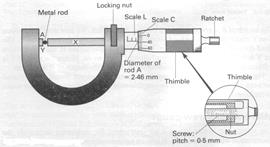
Figure 4.3 – Micrometer gauge
To measure the diameter of a wire or rod A, one side is rested on the end Y and the thimble is turned until the end of the spindle X just touches A. Further turning of the thimble does not make the spindle move forward because a ratchet stops excessive pressure on A. A locking device is sometimes included so that the diameter reading can be read after A is taken out.
An engraved linear millimeters scale L shows the forward movements of the spindle. A circular scale C on the thimble helps to measure fractions of millimeters. In Fig. 4.4, 50 circular divisions = 0.5 mm, or 1 circular division = 0.01 mm. So from Fig. 4.4, diameter of rod = 2.0 mm (scale L) + 46 × 0.01 mm (scale C) = 2.46 mm
Some precautions are needed when using the screw-gauge to measure the diameter of a wire, for example (a) when the gap between X and Y is closed, check if the reading is zero; if not, add or subtract the error to your final reading of the diameter: (b) with the wire in the gap, make a firm but gentle contact with the screw, that is, do not over screw: (c) you should measure the diameter at three different places along the wire to allow for lack of uniformity; at each place, measure two perpendicular diameters to allow for any circular defect and then take the average of the six readings to get the diameter.
4.5 Measurement of mass
The mass of an object can be measured by comparing it with standard masses. These are derived from copies of the International Kilogram Mass. The chemical balance is used to compare masses in this way. Extremely sensitive balances can be designed. The National Physical Laboratory at Teddington in England has a balance which can measure to one-millionth of a gram. At this laboratory very precise weightings are carried out to assist science and technology.
School balances do not require such high precision. The design of chemical balances has altered so much in recent years that the types used will generally differ from school to school. The top pan balance gives direct reading of mass. It does this by comparing the unknown mass with movable known masses inside it. As in the common balance, the unknown and known masses are balanced using a lever arrangement.
If an object is taken say from London, England, to Lagos, Nigeria, or anywhere else in the world, and weighed on any other chemical balance, its mass will be exactly the same. So a chemical balance compares masses.
4.6 Measurement of weight
A spring balance provides a quick method of measuring weight. The object X (fig. 4.4) is hung from the hook and the spring is then pulled out by a length proportional to the weight of X. The weight of X is the force on X due to the earth's gravitational pull. The spring balance has a 'uniform' scale, that is equal divisions represent equal changes in weight along the whole scale. Weights are measured in Newtons (N) in SI units.

Figure 4.4 – Spring balance measuring weight
Unlike the chemical balance, the reading on the spring balance will vary slightly if the same mass is taken to different parts of the world. At the north; for example, it will weigh slightly more than at the equator. This is because gravitational pull is stronger at the poles and so the spring is stretched more. A spring balance measures weight, not mass.
Experimental Part
1. To measure length of the sides of the parallelepiped. One of the sides of it should be measured three times and two other sides are measured one time.
2. To fill results in to the table 4.1.
Table 4.1 – Dates for the parallelepiped
| № | аi, mm | ∆аi, mm | (∆аi )2,mm2 | b, mm | c, mm |
 = ∑ (∆аi )2 =
= ∑ (∆аi )2 =
Table 4.2 – Dates for the cylinder
| № | di, mm | ∆di, mm | (∆di )2,mm2 | h, mm |
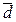 = ∑ (∆di )2 =
= ∑ (∆di )2 =
where ai (di) is the result of measurements;  (
(  ) is the arithmetic mean; ∆аi (∆di) is the accidental error (deviation).
) is the arithmetic mean; ∆аi (∆di) is the accidental error (deviation).
3. To calculate the most probable meaning of density with the formula (4.1).
4. Mean root square:
 (4.6)
(4.6)
5. At first it is necessary to obtain a formula for relative error:
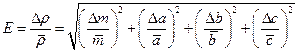 (4.7)
(4.7)
where Δm is the error of a table quantity; Δa, Δb, Δc are half-widthes of the confidencal interval of direct measurements.
6. As we know:
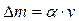 , (4.8)
, (4.8)
where α is a probability; v is a half price of category from last significance figure in a table quantity.
7. As value of a is measured n times ( n = 3 ) we use the formula:
 , (4.9)
, (4.9)
where  is the Student's constant; δ is an error of an instrument.
is the Student's constant; δ is an error of an instrument.
8. Quantities of b and c we obtain by direct measuring and we measure b and c one time ( n =1 ). So we have:
 ,
,  , (4.10)
, (4.10)
where v is an error of count; 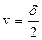 .
.
 9. To calculate quantities of Dm, Da, Db, Dc. Substituting them into formula (4.7), we find E.
9. To calculate quantities of Dm, Da, Db, Dc. Substituting them into formula (4.7), we find E.
10. To find ∆ρ from formula (4.7) in such a way:
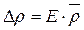 ,
,
where E is the relative error, calculated by formula (4.7);  is the most probable meaning of the substance density, calculated by formula (4.1).
is the most probable meaning of the substance density, calculated by formula (4.1).
11. To write down the result of measurement after approximation using the form:  =
= 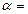 …
… 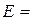 … %.
… %.
Authors: S.P. Lushchin, the reader, candidate of physical and mathematical sciences.
Reviewer: S.V. Loskutov, professor, doctor of physical and mathematical sciences.
Approved by the chair of physics. Protocol № 3 from 01.12.2008.
|
© 2013 wikipage.com.ua - Дякуємо за посилання на wikipage.com.ua | Контакти |